SpiTrex 3D
+1 760-349-1551
1815 Aston Ave #106
Carlsbad, CA 92008
Home | Locations | SpiTrex 3D | SpiTrex 3D Capabilities
Additive Manufacturing, Elevated by Design
At SpiTrex 3D, we deliver a full ecosystem of design, production, and regulatory services driven by orthopedic advancement. Our strength lies in the convergence of Design for Additive Manufacturing (DfAM), engineering expertise, and a fully validated manufacturing environment. From complex titanium implants to high-precision surgical tools, we partner with customers to take products from concept through commercialization with speed, accuracy, and confidence.
With industry leading Renishaw quad-laser printers and an FDA Master File supporting our 3D printing and post-processing methods, we are strategically positioned to accelerate regulatory pathways while maintaining world-class quality and repeatability.

Co-Designing for Additive Manufacturing
We specialize in designing complex implant geometries and surgical tools that unlock the full potential of additive manufacturing. Through enhancements such as optimized lattice structures and integrated surgical features, our co-design process minimizes material usage, improves biomechanical performance, and simplifies production from the outset.
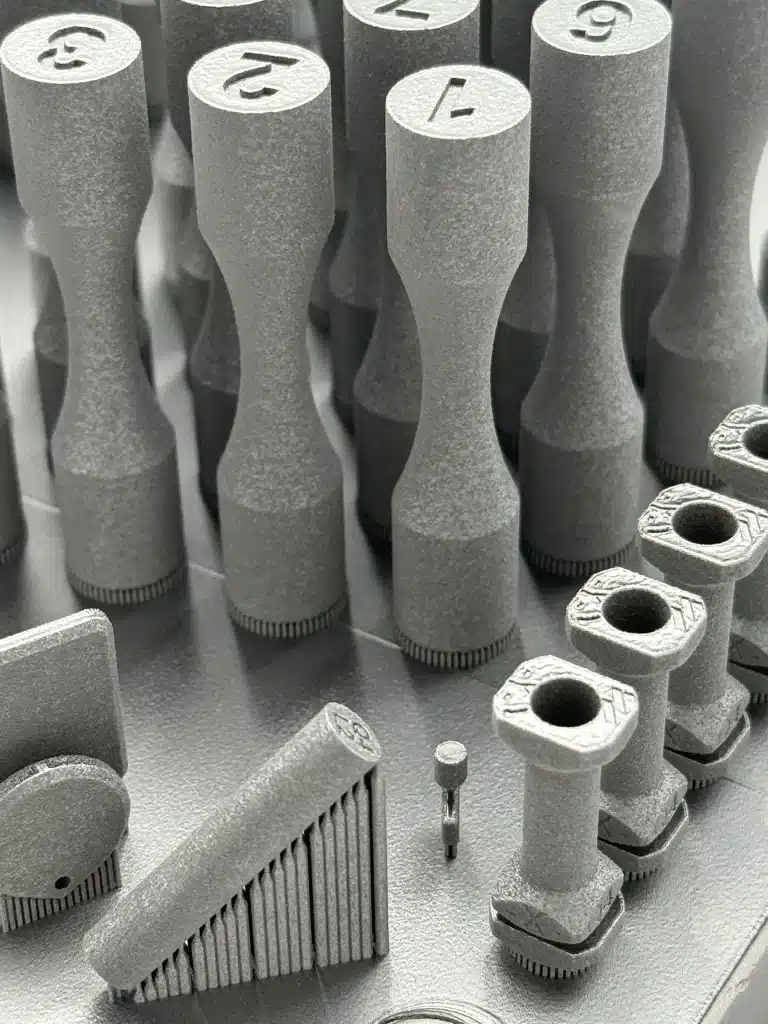
Engineering Services
Our engineering team provides comprehensive DfAM design support, including prototyping, project management, Design History Files, Risk Analyses, and Design Transfer, ensuring each product is development and submission ready.

Additive Manufacturing
We operate a full-scale additive manufacturing platform using best-in-class Renishaw quad-laser systems, delivering consistent, high resolution output for prototypes, verification and validation (V&V) test parts, high volume commercial production, and patient specific devices.
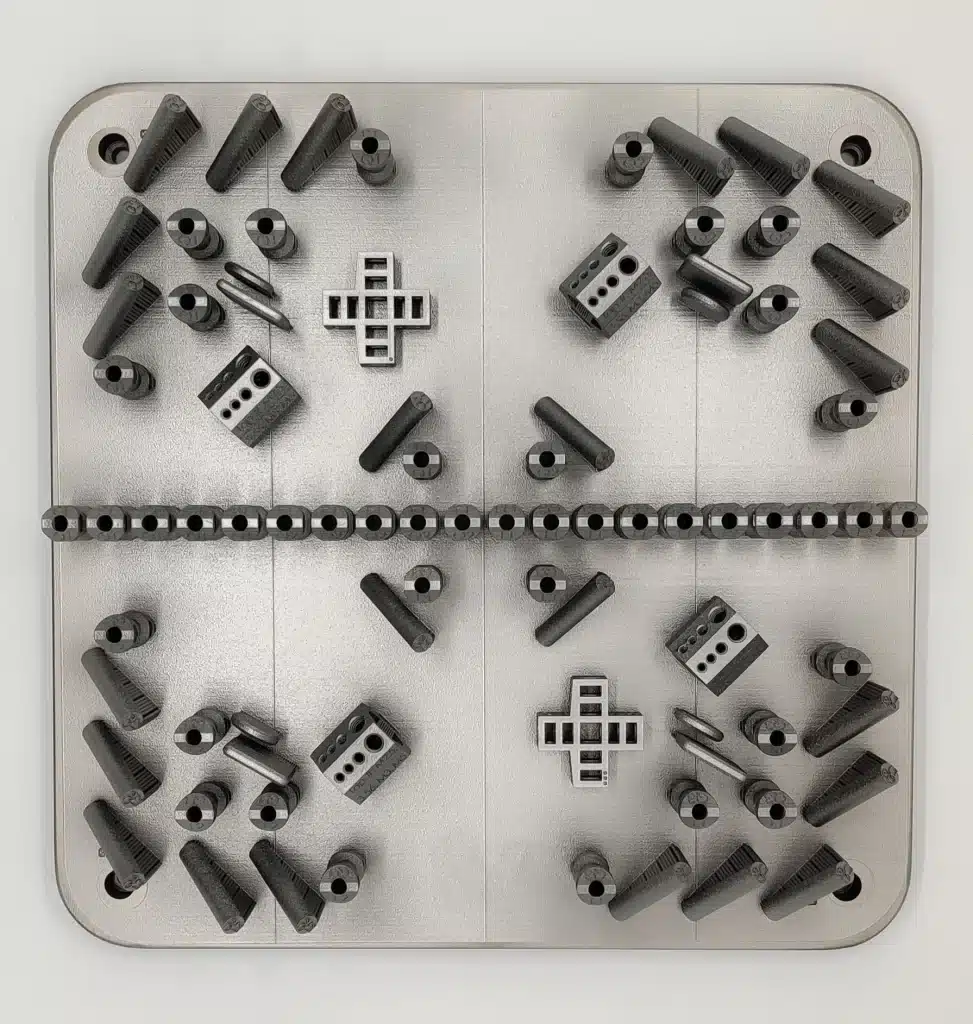
Validations & Regulatory Support
We support every stage of the device lifecycle, including process validations, while assisting with or fully drafting regulatory submissions. Our FDA Master Files streamline new 510(k) submissions and provide justification for transferring existing product lines into our highly controlled manufacturing ecosystem.
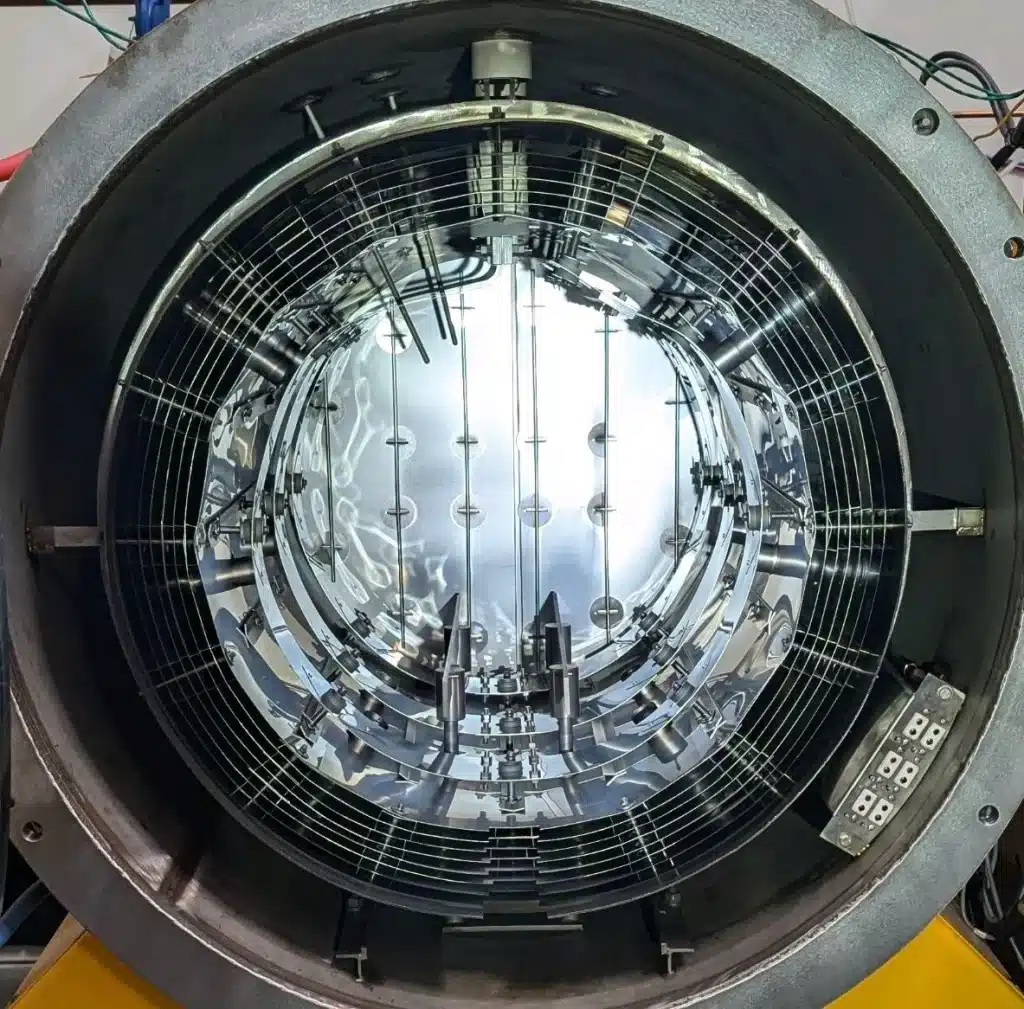
Thermal Treatments, HIP & VSR
We have validated traditional Hot Isostatic Pressing (HIP) and in-house Vacuum Stress Relieving (VSR) cycles to support residual stress relief, microstructure optimization, mechanical property enhancement, and material homogenization.

Subtractive Manufacturing
Our upfront DfAM feedback helps minimize secondary machining, reducing both lead times and costs. When designs require complex subtractive features or assemblies with machined components, our broader SpiTrex locations provide high-precision machining, with workflows fully integrated with additive manufacturing.

Sterile Packaging
Our integrated processes allow us to ship clean and internally passivated 3D-printed products directly to your preferred packaging and sterilization vendor, or we can package and steam sterilize devices in-house within our controlled environment.

Quality Systems
Our ISO 13485-registered and FDA-compliant Quality Management System (QMS) ensures that all products consistently meet customer specifications, regulatory requirements, and applicable safety standards. Our 3D printing process control monitors, fully documented within our FDA Master Files, generate robust statistical datasets that support consistently high-quality products without unnecessarily increasing lead times or costs. Our QMS focus on continual improvement ensures we remain leaders in the rapidly advancing field of additive manufacturing.